Bioclear™ 10 and 11 films are comparable
Bioclear™ 11 is a low-antioxidant film developed as a response to the industry need of improved cell growth in cell-sensitive applications. The film is used in Cellbag™ bioreactors and M*Bag mixing chambers from Cytiva. Bioclear™ 11 is identical to the previous film, Bioclear™ 10, apart from the content of the secondary antioxidant tris(2,4-di-tert-butylphenyl)phosphite (TBPP, often referred to under the trade name Irgafos™ 168). A degradation product of TBPP has been identified as the root cause of impaired cell growth in sensitive cell lines (1). Bioclear™ 11 was therefore developed to have a reduced content of TBPP compared with Bioclear™ 10 film.
We have performed an extensive study to qualify Bioclear™11 for use with Cellbag™ bioreactors and M*Bag mixing chambers. In summary, due to the lower content of TBPP, Cellbag™ bioreactors with Bioclear™ 11 show a greater ability to support TBPP-sensitive Chinese hamster ovary (CHO) cells compared with equivalent Bioclear™ 10 products. Other product requirements regarding mechanical, chemical, and biological properties are met and shown to be fully comparable to products manufactured from Bioclear™ 10 film.
In short, products with Bioclear™ 11 film
- are identical to Bioclear™ 10 products apart from a lower content of the antioxidant TBPP
- show a greater ability to support TBPP-sensitive CHO cells compared with Bioclear™ 10 products
- can easily replace Bioclear™ 10 products
Bioclear™ film grades
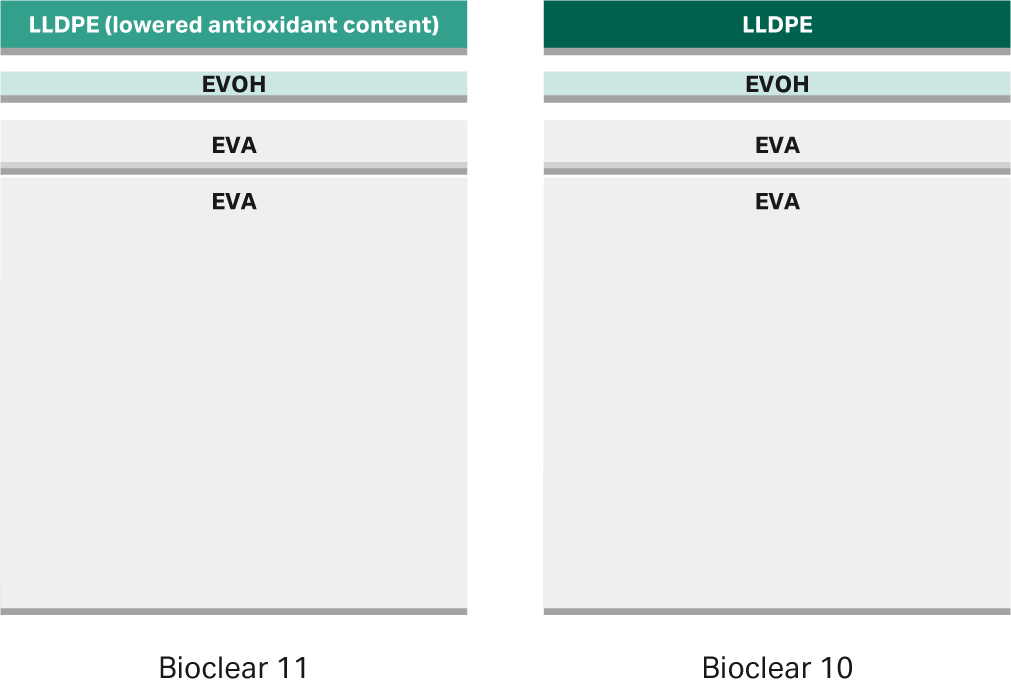
Cellbag™ bioreactors and M*Bag mixing chambers are manufactured from Bioclear™ multilayer polymeric film. Bioclear™ film is available in two grades, Bioclear™ 11 and Bioclear™ 10. The two film grades are identical except for the content of TBPP, which is lower in Bioclear™ 11. The multilayer structure of Bioclear™ 11 and Bioclear™ 10 film is identical (Fig 1) and consists of the following polymers: linear low density polyethylene (LLDPE), poly(ethylene-vinyl alcohol) (EVOH), and poly(ethylene-vinyl acetate) (EVA). It’s been reported that the oxidized form of TBPP undergoes further degradation upon gamma irradiation. One of the degradation products, bis(2,4-di-tert-butylphenyl)phosphate (bDtBPP), has been shown to inhibit growth of some CHO cell lines (1). TBPP is part of the antioxidant package in Bioclear™ 10, and the major source is the LLDPE resin.
Fig 1. Bioclear™ film construction. Schematic of identical layer structure of Bioclear™ 11 and Bioclear™ 10 film. Both films consist of LLDPE (1 mil = 0.001”= 25.4 μm), EVOH gas barrier (0.5 mil), EVA (1 mil), and EVA product contact surface layer (10 mil). The construction and content of the layers are identical apart from a lower antioxidant concentration in the LLDPE layer of Bioclear™ 11.
Bioclear™ 11 film has a lowered content of TBPP and was created by implementing a low-antioxidant grade of the LLDPE resin used in Bioclear™ 10. The two LLDPE resin grades contain the same LLDPE base polymer and are commercially available from the same supplier. Implementation of the LLDPE resin analogue enabled a targeted and controlled method of creating a low-TBPP film, resulting in the Bioclear™ 11 film. The same critical to quality attributes found in Bioclear™ 10 film were maintained without introducing new additives or changing base polymers.
Summary of results
Cell growth
The improved cell growth performance for Bioclear™ 11 Cellbag™ bioreactors was verified by using a CHO DG44 cell line (licensed from Cellca GmbH, Laupheim, Germany). The cell line was maintained in ActiCHO™ SM medium in 125 mL shake flasks on a rotary shaker at 100 rpm placed in a humidified incubator at 36.8°C and 7.5% CO2. The cultures were split every third or fourth day to an initial viable cell density (VCD) of 0.3 × 106 cells/mL. Glutamine was added to the medium to a final concentration of 6 mM.
Sensitivity to bDtBPP
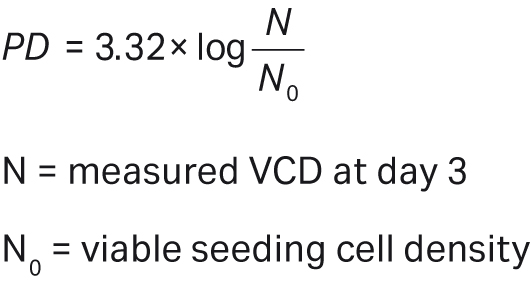
The cell line’s sensitivity to bDtBPP was verified by spiking ActiCHO™ SM medium with known concentrations of bDtBPP. The growth-promoting capacity of the respective spiked medium samples was assessed in triplicate 25 mL shake flask cultures. The cell inoculum was taken from maintenance cultures with a VCD of 3.5 to 5 × 106 cells/mL. The cells were in passage 3 to 30 post-thaw. The inoculum was centrifuged at 400 × g for five min. After centrifugation the supernatant was discarded, and the cell pellet suspended in 37°C prewarmed test and control medium to a start cell concentration of 0.3 × 106 cells/mL. The VCD was determined using a CASY™ TT cell counter (Roche Diagnostics, Switzerland) after three days. The number of population doublings during three days (PD) was calculated from the following equation:
N = measured VCD at day three
N0 = viable seeding cell density
The cell growth performance was calculated according to:
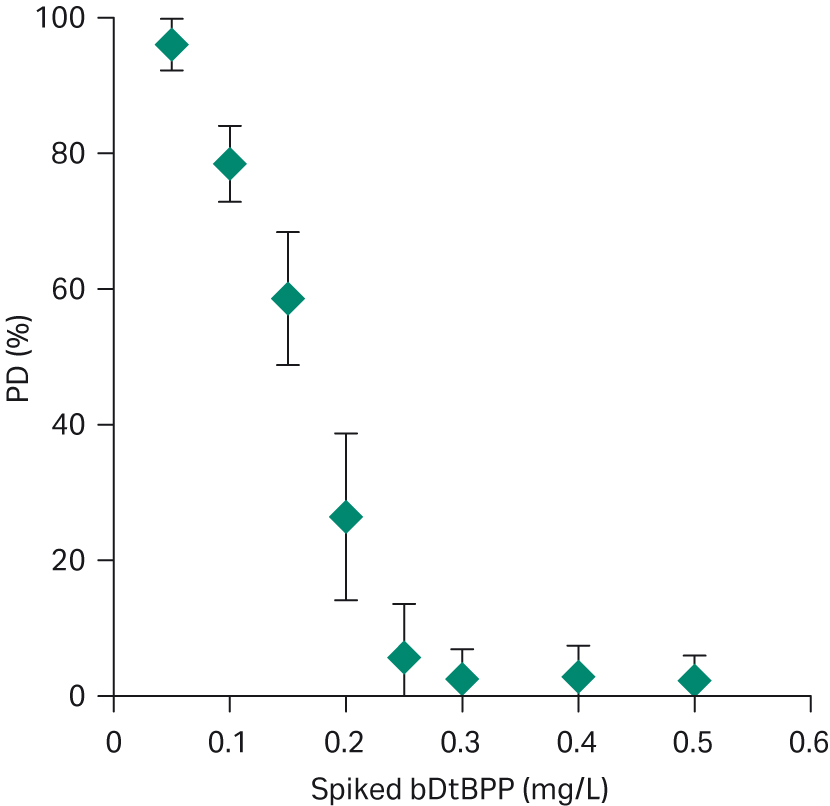
As shown in Figure 2, the CHO cell line detected levels of bDtBPP down to 0.1 mg/L (100 ppb) which is consistent with results reported for other bDtBPP-sensitive cell lines (1).
Fig 2. Correlation of cell growth to concentration of bDtBPP spiked into cell culture medium.
Verification of cell growth
The CHO DG44 cell line was used to verify the improved cell growth performance of Bioclear™ 11 using sterilized 2 L Cellbag™ bioreactors according to the procedure outlined below. The Cellbag™ bioreactor was inflated and mounted to a WAVE Bioreactor™ 20/50 system and filled with 200 mL ActiCHO™ medium. With all ports clamp-sealed, the Cellbag™ bioreactor was set to rock at a speed of 20 rpm, an angle of six degrees, and a temperature of 37°C. The temperature control on the WAVE Bioreactor™ system was switched off after three days. The rocking was stopped at day seven. These medium incubation conditions correspond to approximately 8 cm2 of film surface area/mL medium and were chosen to represent a worst-case scenario to aggressively challenge the film performance. After incubation, the medium was harvested, 80 mL of the incubated medium was added to a T-flask, and cell growth of the bag extract was assessed according to the same procedure as described for the sensitivity study. The cell growth performance was calculated according to
Variability studies
Cellbag™ bioreactors consisting of two lots of Bioclear™ 11 film were evaluated for cell growth performance. Tests were performed in less than eight weeks after gamma sterilization. Both a nominal gamma dose and a higher gamma radiation dose targeting 42.5 to 55 kGy were evaluated. Cell growth performance was also evaluated after periods of accelerated aging corresponding to one and two years real-time aging at 22°C (for details on aging conditions, see Shelf life section).
Cell growth intra-lot variability was evaluated by using multiple rolls of each Bioclear™ 11 film lot. A total of 14 film rolls were tested. For the study, 2 L Cellbag™ bioreactors were manufactured using different rolls of the polished/matte film at the bottom side of the bioreactors. Film roll numbers are indicated in Table 1. Cell culture results are compiled in Table 1 and Figure 3. Each data point is an average of two tested Cellbag™ bioreactors.
Table 1. Cell growth test results for Bioclear™ 11 film using a CHO DG44 monoclonal antibody-producing cell line
| Bioclear™ 11 |
Number of days post gamma irradiation |
PD (%) |
Viability (%) |
Comments |
| Lot # T130130/T130135 | ||||
| Film roll number | ||||
| 1 | 6 | 98 | 94 | |
| 5 | 6 | 98 | 94 | |
| 10 | 6 | 99 | 94 | |
| 16 | 13 | 91 | 94 | |
| 18 | 13 | 89 | 95 | |
| 18 | 23 | 93 | 94 | |
| 18 | 52 | 95 | 93 | |
| 18 | 138 | 98 | 95 | Accelerated aging at 50°C simulating one year |
| 18 | 138 | 96 | 95 | Accelerated aging at 50°C simulating one year |
| 18 | 201 | 93 | 95 | Accelerated aging at 50°C simulating one year |
| 18 | 170 | 98 | 94 | Accelerated aging at 50°C simulating one year |
| 18 | 16 | 98 | 94 | High gamma dose targeting 42.5-55 kGy |
| Lot # 140923/140924 | ||||
| Film roll number | ||||
| 1 | 6 | 95 | 94 | |
| 12 | 7 | 99 | 93 | |
| 24 | 6 | 97 | 94 | |
| 29 | 6 | 94 | 94 | |
| 29 | 9 | 98 | 94 | |
| 41 | 6 | 95 | 94 | |
| 53 | 6 | 96 | 94 | |
| 57 | 7 | 98 | 93 | |
| 68 | 9 | 99 | 93 | |
| 80 | 6 | 96 | 94 | |
| 1 | 9 | 94 | 95 | High gamma dose targeting 42.5-55 kGy |
| 29 | 9 | 93 | 95 | High gamma dose targeting 42.5-55 kGy |
| 57 | 9 | 92 | 95 | High gamma dose targeting 42.5-55 kGy |
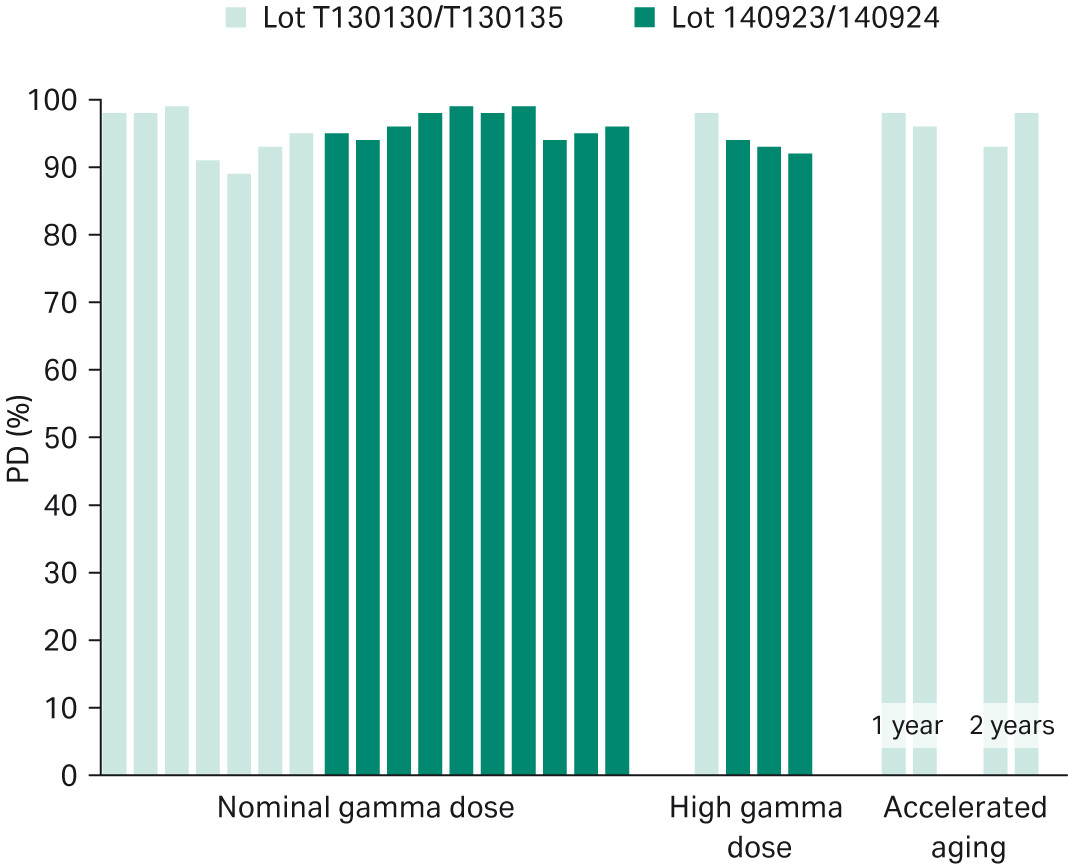
As seen in Figure 3, Bioclear™ 11 supported consistent cell growth in the range of 89% to 99% PD relative to a control. Average cell growth was determined to 96% PD with a standard deviation of 3% PD. Figure 3 also verifies that the high cell growth performance is maintained after high gamma dose treatment at 42.5 to 55 kGy (94% PD, standard deviation 2% PD) as well as after accelerated aging at 50°C for a time period simulating two years real-time aging at 22°C (96% PD, standard deviation 3% PD).
Fig 3. Cell growth of Bioclear™ 11 film ≤ 2 months after nominal gamma dose and high gamma dose. Cell growth of Bioclear™ 11 film after accelerated aging simulating one year and two years real-time aging. Each bar represents an average of two Cellbag™ bioreactors tested.
Extractables
An extractables study was performed on 2 L Bioclear™ 11 Cellbag™ bioreactors exposed to a high gamma dose of 42.5 kGy to 55 kGy, exceeding the normal dose of 27.5 kGy to 40 kGy. Extractions were carried out under exaggerated conditions, compared with normal use, regarding polarity of the solvents, pH, temperature, and contact time (Table 2). The Cellbag™ bioreactors were filled with 1 L extraction solution for four weeks at 50°C. After extraction, the extracts were analyzed with a range of analytical techniques to characterize volatile, semi-volatile, and non-volatile organic compounds, as well as elements, ions, total organic carbon, pH, conductivity, and non-volatile residue.
The results of the extractables study of Bioclear™ 11 Cellbag™ bioreactors was compared with previously performed studies on the equivalent Bioclear™ 10 products (Table 2). Detailed results of each Cellbag™ variant are reported in the validation guides for Cellbag™ bioreactors and M*Bag mixing chambers. A comparison of the extractables profiles is shown in Table 3. As some extractables are specific to the perfusion filter, the profile of Bioclear™ 11 products was primarily compared to the profile of Bioclear™ 10 products with pHOPT and DOOPT sensors.
In summary, for both Cellbag™ variants, the main extractables were acetate and formate leached from the EVA layers of the film. Other extractables identified in both variants were related to lubricants/slip agents, antiblock agents, antioxidants, and processing aids. The same additives are used in Bioclear™ 11 and Bioclear™ 10, except for the lower antioxidant level in Bioclear™ 11 film. Regarding antioxidants, Table 3 shows that the phenolic antioxidant degradation product 7,9-di-tert-butyl-1-oxaspiro(4,5)deca-6,9-diene-2,8-dione was leached to a comparable extent from Bioclear™ 11 and Bioclear™ 10 products. In contrast, the organophosphite antioxidant degradation product bDtBPP was leached to a lower extent from Bioclear™ 11 film due to the removal of TBPP from the LLDPE resin. A comparison between leached levels of bDtBPP is shown in Table 4.
As can be seen in Table 3, several carboxylic acids were identified in all Cellbag™ variants. The carbon chain lengths range from C6 (hexanoic acid) to C18 (stearic acid, oleic acid). These are likely slip agent degradation products. The reason for the variability in fatty acids extractables profile between the studies is under investigation.
Table 2. Test conditions for extractables studies performed on 2 L Cellbag™ bioreactors
| Cellbag™ bioreactors |
CB0002L11-31: Bioclear™ 11 film, pHOPT and DOOPT sensors |
X-CB0002L11-01 Bioclear™ 11 film, X-CB0002L10-01 Bioclear™ 10 film, |
X-CB0002L31-02: Bioclear™ 10 film, pHOPT and DOOPT sensors, Oxywell CB0002L10-02: Bioclear™ 10 film, No Sensors |
CB0002L10-04: Bioclear™ 10 film, Perfusion filter, Replacement of Oxywell with Tempwell |
| Date of study | Jan – Apr 2014 | Feb – Sep 2015 | Nov 2011 – Feb 2012 | Jul – Nov 2013 |
| Gamma irradiation | 42.5 – 55 kGy | 2 x 27.5-40 kGy | 2 x 27.5-40 kGy | 42.5 – 55 kGy |
| Extraction start | < six weeks post gamma irradiation | seven weeks post gamma irradiation | < four weeks post gamma irradiation | < two weeks post gamma irradiation |
| Extraction time | 28 days | 28 days | 28 days | 28 days |
| Temperature | 50°C | 50°C | 50°C | 50°C |
| Relative humidity | 75% | 75% | Not specified | Not specified |
| Volume | 1.0 liter | 1.0 liter | 1.0 liter | 1.0 liter |
| Film surface area | 2 × 838 cm2 | 2 × 838 cm2 | 2 × 838 cm2 | 2 × 838 cm2 |
| Area/volume ratio | 1.7 cm2/mL | 1.7 cm2/mL | 1.7 cm2/mL | 1.7 cm2/mL |
| Extraction mode | Static | Static | Static | Static |
| Solvents | WFI | PBS pH 9 | WFI | WFI |
| PBS pH 5 | PBS pH 5 | PBS pH 5 | ||
| PBS pH 9 | PBS pH 9 | PBS pH 9 | ||
| 20% Ethanol | 20% Ethanol | 20% Ethanol | ||
| 1% Kolliphor P188 | 1% Kolliphor P188 |
Table 3. Comparison of identified extractables (ng/cm2) leached from Bioclear™ 11 Cellbag™ bioreactors (BC11) with HOPT/DOOPT sensors and Bioclear™ 10 (BC10) with pHOPT/DOOPT sensors
| WFI (ng/cm2) |
PBS pH 5 (ng/cm2) |
PBS pH 9 (ng/cm2) |
Non-volatiles PBS pH 9 (ng/cm2) | 20% EtOH (ng/cm2) | 1% Kolliphor (ng/cm2) | |||||||
| BC11 | BC10 | BC11 | BC10 | BC11 | BC10 | BC11 | BC10 | BC11 | BC10 | BC11 | BC10 | |
| Anions | ||||||||||||
| Acetate | 5600 | 12000 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| Acetate | 5600 | 12000 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| Chloride | <30 | <600 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| Formate | 609 | 1492 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| Nitrate | <30 | <600 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| Phosphate | 119 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| Sulphate | 30* | <600 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| Elements | ||||||||||||
| Al | <2 | 5 | <2 | <2 | <2 | <2 | n.a. | n.a. | n.a. | <89 | n.a. | n.a. |
| Ca | 129 | 341 | 13 | 38 | (6) | 18. | n.a. | n.a. | n.a. | 500 | n.a. | n.a. |
| K | (24) | 318 | 12 | 136 | (119) | 196 | n.a. | n.a. | n.a. | <537 | n.a. | n.a. |
| Mg | 17 | 34 | 2 | 3 | <2 | <2 | n.a. | n.a. | n.a. | <89 | n.a. | n.a. |
| Na | n.a. | 324 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | (146) | n.a. | n.a. |
| S | 54 | n.a. | 6 | n.a. | (18) | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| Si | 149 | 200 | (101) | 177 | (149) | 180 | n.a. | n.a. | n.a. | <418 | n.a. | n.a. |
| Zn | 0.6 | 8 | 0.6 | 2 | <0.6 | 0.6 | n.a. | n.a. | n.a. | <30 | n.a. | n.a. |
| Organic compounds | ||||||||||||
| 2-ethyl-1-hexanol | 16 | 9 | 16 | 9 | 15 | 7 | n.a. | n.a. | 60 | - | - | - |
| 2-ethylhexanoic acid | - | - | - | - | 4 | 5 | n.a. | n.a. | - | - | - | - |
| 2,4-dichlorobenzoic acid | - | - | - | - | - | - | n.a. | n.a. | 43 | - | - | - |
| 7,9-di-tert-butyl-1-oxaspiro(4,5)deca-6,9-diene-2,8-dione | 4 | - | - | - | 11 | 8 | n.a. | n.a. | - | - | - | - |
| Acetaldehyde | - | - | - | - | - | - | n.a. | n.a. | 8 | 5 | 119 | 286 |
| bDtBPP | - | - | - | 5 | 10 | 40-250* | 16 | 137 | - | - | - | - |
| Benzoic acid | 119 | 7 | - | 4 | 14 | 4 | n.a. | n.a. | 30 | - | - | - |
| Caprolactam | 4 | 4 | 7 | 4 | - | - | - | - | - | - | - | - |
| Decanoic acid | - | - | - | - | - | 10* | n.a. | n.a. | - | - | - | - |
| Dodecanoic (lauric) acid | - | - | - | - | 4 | 14* | n.a. | n.a. | - | - | - | - |
| Ethyl acetate | - | - | - | - | - | - | n.a. | n.a. | 33 | 38 | - | - |
| Hexadecanoic (palmitic) acid | - | - | - | - | 149 | 4* | 141 | 91 | - | - | - | - |
| Hexanoic | - | - | - | - | - | 6* | n.a. | n.a. | - | - | - | - |
| Heptanoic | - | - | - | - | - | 6* | n.a. | n.a. | - | - | - | - |
| Isopropanol | - | - | - | - | - | - | n.a. | n.a. | - | 5 | - | - |
| Myristic acid | - | - | - | - | 60 | - | 17 | 33 | - | - | - | - |
| N-(1-Cyano-1-methylethyl)isobutyro amide | - | 4 | - | 5 | - | 4 | n.a. | n.a. | - | - | - | - |
| Nonanoic (pelargonic) acid | - | - | - | - | 13 | 45* | n.a. | n.a. | - | - | - | - |
| Octadecanoic (stearic) acid | - | - | - | - | 29 | - | 14 | 19 | - | - | - | - |
| Octanoic (caprylic) acid | - | - | - | - | 4 | 13* | n.a. | n.a. | - | - | - | - |
| Oleic acid | - | - | - | - | 28 | - | 18 | 16 | - | - | - | - |
| Palmitoelic acid | - | - | - | - | 8 | - | - | - | - | - | - | - |
| Pentadecanoic acid | - | - | - | - | 14 | - | 4.9 | 7.6 | - | - | - | - |
| Undecanoic | - | - | - | - | - | 3* | n.a. | n.a. | - | - | - | - |
n.a. = not analyzed.
() only indicative because blank value was in the same range or higher than the measured value.
*Quantitative results based on extractables study of Cellbag™ bioreactors with perfusion filter. The analytical equipment for HPLC-MS analysis was updated in between the extractables study of Bioclear™ 10 Cellbag™ bioreactors with sensors and the two later studies (Bioclear™ 10 with perfusion filter and Bioclear™ 11, respectively).
For details on detection limits see validation guides for Cellbag™ bioreactors and M*Bag mixing chambers.
Table 4. Comparison of bDtBPP leached from 2 L Cellbag™ bioreactors
| Cellbag™ film | WFI (ng/cm2) | PBS pH 5 (ng/cm2) | PBS pH 9 (ng/cm2) | 20% EtOH (ng/cm2) | 1% Kolliphor (ng/cm2) |
| Bioclear™ 11 | n.d. | n.d. | n.d. | n.d. | n.d. |
| Bioclear™ 10 | n.d. | 5 | 137 | n.d. | n.d. |
n.d. = not detectable.
Film area/volume ratio: 1.7 cm2/mL.
Note: The reporting limits are 0.5 mg/L (300 ng/cm2) for 1% surfactant, 0.1 mg/L (60 ng/cm2) for 20% ethanol, and 0.005 mg/L (3 ng/cm2) for the other solvents.
Quantification was done using an internal standard of Tinuvin™ 327 and the relative response factor for bDtBPP was assumed to be 1.
A targeted study was performed to further study the leaching of bDtBPP. Cellbag™ bioreactors with Bioclear™ 11 and Bioclear™ 10 were each filled with 200 mL ActiCHO™ SM medium, which was incubated in the bags for seven days on WAVE Bioreactor™ 20/50 system at incubation and surface area to volume settings equal to settings used during cell growth assessment (see Cell growth section). As shown in Table 5, Bioclear™ 11 Cellbag™ bioreactors leached lower levels of bDtBPP. When comparing Table 4 and Table 5, it can be concluded that, of the solvents used in the extractables study, PBS pH 9 provides the most relevant conditions for modelling exaggerated extraction of bDtBPP into cell culture medium.
Table 5. Comparison of bDtBPP leached from 2 L Cellbag™ bioreactors expressed in units mg/L and ng/cm2
| Cellbag™ film | ActiCHO™ SM (mg/L) | ActiCHO™ SM (ng/cm2) |
| Bioclear™ 11 | 0.05 | 6 |
| Bioclear™ 10 | 0.2-0.8 | 25-95 |
Film area/volume ratio: 8.4 cm2/mL
Note: The reporting limit is 0.010 mg/L (1.2 ng/cm2). Quantification was done using a calibration curve of bDtBPP standards.
Shelf life
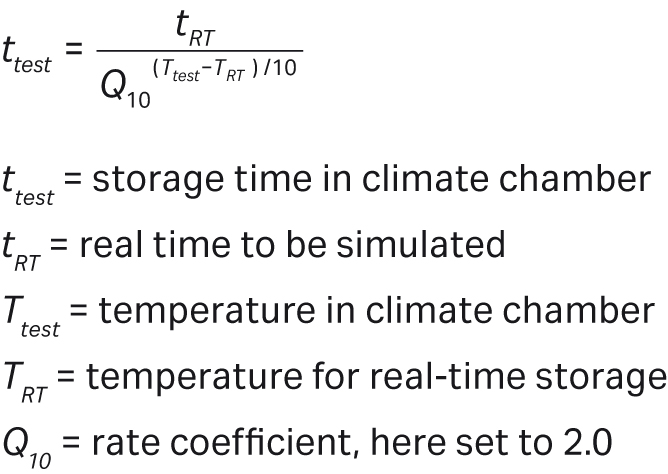
Bioclear™ 11 Cellbag™ bioreactors have a shelf life of two years, based on data generated in an accelerated aging study. A real-time aging study has been initiated. Bioclear™ 10 Cellbag™ bioreactors have a shelf life of two years. In the accelerated aging study, Bioclear™ 11 Cellbag™ bioreactors and M*Bag mixing cambers were incubated at 50°C •} 5°C, non-specified humidity, for a time period corresponding to two years real-time aging at 22°C based on the Arrhenius equation.
Mechanical properties
There’s no change in form, fit, or function of Bioclear™ 11 Cellbag™ bioreactors compared to the Bioclear™ 10 equivalent. The drawings for both Bioclear™ 11 Cellbag™ bioreactors and M*Bag mixing chambers are identical to corresponding bag drawings for Bioclear™ 10 products, except for the film identity. All assembly parts, such as tubings and ports, are identical in bags of Bioclear™ 11 and Bioclear™ 10. Bags offered with Bioclear™ 11 film range from 2 to 200 L and are listed in the data file "Disposable Cellbag™ bioreactors for WAVE Bioreactor™ systems" (28951136).
Film physical properties
Physical properties of Bioclear™ 11 are comparable to those of Bioclear™ 10 film. Values for both films are listed in Table 6.
Table 6. Typical test results of physical properties of Bioclear™ 11 and Bioclear™ 10 film
| Physical property | Procedure | Unit of measure | Bioclear™ 11 | Bioclear™ 10 |
| Tensile strength at break | ASTM D-882 | MPa | 21 | 21 |
| Elongation at break*** | ASTM D-882 | % | >500 | >500 |
| Tensile E-Modulus | ASTM D-882 | MPa | 116 | 116 |
| Low temp. brittleness | ASTM D-1790 | °C | ≤ -45 | ≤ -45 |
| Haze | ASTM D-1003 | % | 81 (matte), 8 (polished) | 83 (matte), 8 (polished) |
| Clarity | ASTM D-1003 | % | 12 (matte), 90 (polished) | 11 (matte), 90 (polished) |
| Transmittance | ASTM D-1003 | % | 15 (matte), 80 (polished) | 15 (matte), 80 (polished) |
| O2 permeability | ASTM D-3985 | cm3/100in2/24hrs @25°C | 0.28* | 0.28 |
| CO2 permeability | ASTM F-2476 | cm3/100in2/24hrs @25°C | 0.58* | 0.58 |
| Water vapor transmission rate | ASTM F-1249 | gms./100in2/24hrs @25°C 100%RH | 0.11* | 0.11 |
* Test data based on Bioclear™ 10 film. Equivalent results are expected as the EVOH barrier is identical in the two films.
† 1 MPa = 10 bar = 145 psi
Seal strength
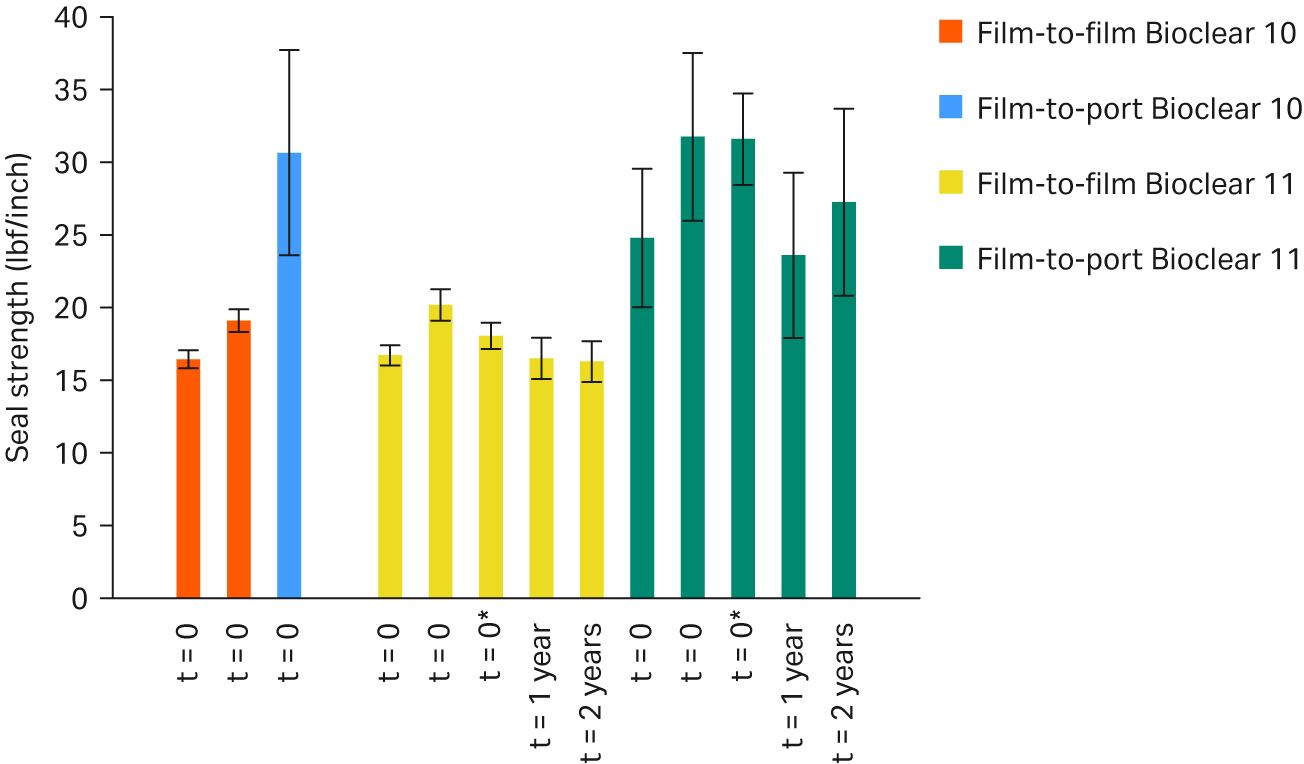
The strength of Bioclear™ 11 and Bioclear™ 10 film heat seal bonds was evaluated and was shown to be substantially equivalent based on statistical analysis of pull test data (Fig 4).
Fig 4. Seal strength of Bioclear™ 11 film-to-film and film-to-port tested after gamma irradiation at 27.5kGy to 40 kGy (t = 0), after gamma irradiation at 42.5 kGy to 55 kGy (t = 0*), and after gamma irradiation at 27.5 to 40 kGy followed by accelerated aging at 50°C corresponding to one year and two years real-time aging at 22°C. Each bar contains an average of approximately 30 samples.
The failure mode of both films was the same for all tests. The heat seal strength results are in line with the understanding that the physical properties of the two films are substantially equivalent.
Pharmacopeia and ISO 10993 conformance
Bioclear™ 11 film and Bioclear™ 10 film conform to the following tests
- USP class VI (USP <88>) for plastics
- USP <87> Cytotoxicity
- USP <661> Physicochemical tests for plastics
- USP <85> Bacterial Endotoxins – LAL*
- European Pharmacopeia 3.1.7*
- ISO 10993-4 Hemolysis
- ISO 10993-5 Cytotoxicity
* Compilation of test data for Bioclear™ 11 film are in process. Test results are expected to be equivalent to Bioclear™ 10 as both films share the same suppliers, manufacturing conditions, base polymers, and additives except for the lower content of TBPP in Bioclear™ 11.
Animal origin
The polyolefinic formulations used in Bioclear™ 11 and Bioclear™ 10 film both comply with the European legislation on transmissible spongiform encephalopathies (TSE) risk reduction:
- European Pharmacopoeia 8th edition, chapter 5.2.8., monograph 1483
- EMA/410/01 Rev.3 section 6.4
Sterility
AAMI Standard TIR 33, Sterilization of healthcare products—Radiation—Substantiation of a selected sterilization dose— Method VDmax, is a guide for initiating and maintaining a sterile product claim. Together with the standard ISO 11137 Sterilization of health care products, it provides general guidance to facilitate the process of defining a product family. Factors considered for defining a family are
- Nature and sources of raw materials, including the effect, if any, of raw materials that might be sourced from more than one location
- Components
- Product design and size
- Manufacturing process
- Manufacturing equipment
- Manufacturing environment
- Manufacturing location
Based on these factors, Bioclear™ 10 and Bioclear™ 11 Cellbag™ bioreactors are considered as one product family, as the only difference is the lower antioxidant content in Bioclear™ 11. They are also considered equivalent from the perspective of bioburden risk
Supply chain control
We take great care in securing the supply chain for our products. To ensure high and consistent quality of products manufactured from Bioclear™ 11 film, a number of raw material controls are in place throughout the supply chain as described below.
Film supplier qualification
Our supplier has been qualified according to Cytiva purchasing controls procedures.
Raw material agreements
The following raw material agreements are in place:
- A change notification agreement with the film supplier
- A no-change agreement between the film supplier and the supplier of the LLDPE/EVOH/EVA laminate
Inventory
Inventory management is part of the strategy for securing long-term availability of Bioclear™ 11 film.
Controls related to TBPP
As part of Bioclear™ 11 qualification, raw material management procedures were implemented to ensure that Bioclear™ 11 film is only manufactured from LLDPE/EVOH/EVA laminate of consistently low levels of oxidized TBPP to sustain high cell growth.
Conclusions
Bioclear™ 11 film was derived from Bioclear™ 10 film with the explicit intent of reducing TBPP levels while making no other changes to the composition or manufacturing process of the film. Qualification data demonstrate that Cellbag™ bioreactors made from Bioclear™ 11 film support cell growth and that product requirements regarding mechanical, chemical, and biological properties are met and shown to be fully comparable to products manufactured from Bioclear™ 10 film. The test results are also relevant for M*Bag mixing chambers, as these products are made from the same film grades as Cellbag™ bioreactors.
The validation guides for Cellbag™ bioreactors and M*Bag mixing chambers will support you further in making a well-informed technical decision as to the validation and qualification work involved in adopting Bioclear™ 11. There are two validation guides available, one for products using Bioclear™ 11 and one for products using Bioclear™ 10.
References
- Hammond M. et al. Identification of a leachable compound detrimental to cell growth in single-use bioprocess containers PDA J. Pharm. Sci. and Technol. 2013;67: 123–134 .
Related literature
| Validation guides | Product code |
| Cellbag™ bioreactors and M*Bag mixing bags with Bioclear™ 11 film | 11003009 |
| Cellbag™ bioreactor and M*Bag mixing bag with Bioclear™ 10 film for the WAVE Bioreactor™ system | 28960655 |