Article inspired by a Tapas and TechTalks digital event. Fast Trak Centre for Advanced Therapeutic Cell Technologies (CATCT) team members shared insights from customer collaborations.
Lentiviral vectors (LVV) are a common vehicle to deliver genetic material in CAR T cell therapy and gene therapy applications. Production methods have been developed by adapting technologies from the bioprocessing sector. However, these downstream workflows are long, require substantial manual labor, and suffer from low yields of infectious virus. In this article we discuss process development pain points of a modern workflow and ways to address them. We look at new technologies that may be applicable to cell and gene therapy as well as older technologies that have proven very valuable in bioprocessing and can be adapted to serve similar functions in cell and gene manufacturing.
Scale-up of traditional processes
Many classical methods for downstream LVV processing include ultracentrifugation, which is not scalable. Legacy purification methods from bioprocessing of vaccines and proteins, such as monoclonal antibodies, are often used, because specific solutions for lentivirus vectors are not available. These classical methods are time consuming and labor intensive. They also tend to include multiple steps that are open to the environment, posing the risk of microbial contamination.
Designing a downstream process
The aim of any process developer is to meet the current industry standard of a downstream LVV process that is scalable, single-use, and closed to achieve viral vector recoveries of more than 30%, which is the current industry standard. In this case study we are balancing this requirement with a goal to achieve a concentration of 1 × 108 infectious titer/mL with 2- to 3-log removal of contaminants such as host cell protein and host cell DNA. Ideally, we will design a process where the whole downstream workflow could be completed within one day (i.e. limiting the process time to less than eight hours at room temperature.)
Modern ways to address industry pain points
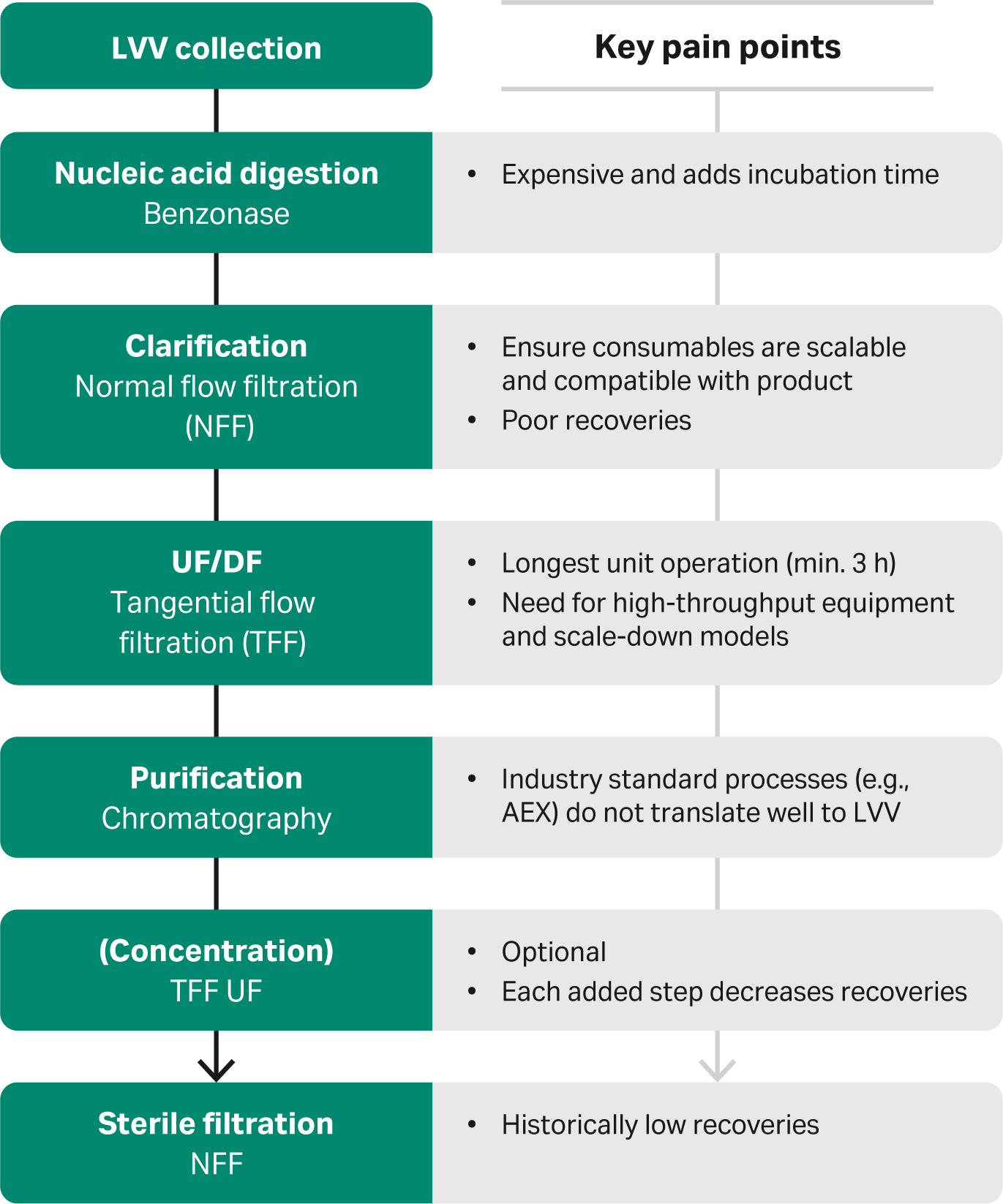
Steps and key pain points in a modern process are highlighted in Figure 1.
Fig 1. Steps in a modern manufacturing process for downstream LVV processing, including key pain points.
In a modern process, lentiviral vector is collected from upstream; in this case, we use a suspension culture in a serum-free medium. Then, nucleic acids are digested using Benzonase™. This enzyme is quite expensive and adds incubation time to a day that is already very long. Next is clarification, which includes removing cellular debris through normal flow filtration (NFF).
Tip: Here it is important to ensure consumables are compatible with the product and are scalable. Some groups have reported difficulties achieving high viral recoveries from this step.
Next is ultrafiltration/diafiltration (UF/DF), which concentrates the virus and exchanges the buffer using tangential flow filtration (TFF). TFF is by far the longest step of the entire downstream process workflow. It requires at least three hours at a one-liter scale.
Following that is purification by chromatography. In contrast to adeno-associated virus (AAV), there is no specific affinity purification method for LVV. The industry has been using anion exchange (AEX), but this doesn’t translate well to LVV. Anion exchange requires high salt concentrations to elute the virus, which the virus doesn’t tolerate well.
After that is an optional concentration step for groups that require a higher concentration of the virus. But it’s important to note that every added step in the downstream process can reduce viral recoveries. Sterile filtration is at the end of the workflow. Groups across the board have recorded historically low recoveries at this step.
In addition to the pain points in the downstream process, there are challenges with the virus itself. Lentivirus buds off from the host cell as an enveloped virus, and the virus must be physically separated from the host cells. LVV requires a very narrow range of pH, temperature, shear stress, salt concentration, and osmolality. In addition, the virus is prone to degradation at room temperature. On top of that, many consumables in a downstream processing platform are not specific for LVV.
Optimizing the downstream process
Before describing how we optimized the process, it’s important to emphasize the key role of analytics in evaluating our results. Locking down the analytics early on was critical, so we could use the same method of measurement across all stages of our process development efforts. And, we found that there are merits in automating a lot of the analyses, including using a liquid handler to make dilutions for all the assays and using plate washers for all the enzyme-linked immunosorbent assays (ELISAs). Also, using a robot is great for reproducibility. It minimizes human error and frees up time for our staff to do other things like data analysis.
Tip: For the gene transfer assay that measures infectious titer, look at variability between operators during cell seeding and gating the flow cytometer. This also translates to variability in the rest of the assays.
Example of an optimized process – 1 L LVV downstream workflow
A summary of our optimization work is provided in Table 1.
Briefly, a 15-minute incubation with Benzonase at room temperature was sufficient to degrade the majority of DNA below 200 bp, to meet the regulatory requirement of no open reading frames (ORF). We identified scalable and compatible consumables for the clarification step and fine-tuned this step to maximize virus recovery. For the first UF/DF step we achieved good recovery and clearance of both protein and DNA from host cells. Also, we identified high-throughput technology and a suitable scale-down model for process development. For purification by chromatography, we developed a multimodal chromatography (MMC) step that combines size exclusion and anion exchange properties . Importantly, the MMC step is simple and processing is gentle. For the final sterile filtration step we maximized recovery by optimizing filter material, layers, and other factors.
Table 1. Summary of process development and outcomes for a 1 L LVV DSP workflow
| Description | Benzonase | Normal flow filtration (NFF) | Tangential flow filtration (TFF) UF/DF | Chromatography | TFF UF | NFF |
|---|---|---|---|---|---|---|
| Variables investigated | Concentration, incubation time, temperature | Pore size, material, filter area, LMH, number of filters | Pore size, filter area, shear rate | Resin, flow rates, load/elute buffers | Pore size, filter area, shear rate | Filter configuration, loading, LMH |
| Step recoveries achieved | 106.3% ± 11.9% (n=19) | 48.6% ± 10.2% (last 30 experiments) | 58.3% ± 10.5% (last 10 experiments w/o known issues) | 80%–95% | 70.1% ± 17.9% (last 7 experiments with current parameters) | |
| Host cell protein clearance | 80%–95% | 80%–95% | ||||
| Host cell DNA clearance | 70%–90% | 80%–95% | ||||
LMH = liters/m2/h
Overall best practices for downstream viral vector process development
Tip: How the virus is produced upstream can greatly impact the downstream process.
For example, our suspension process generates much higher cell densities than our adherent process does, which poses challenges for the filtration steps. In addition, these higher cell densities bring higher host cell protein and DNA contaminants that must be removed. Another thing to consider is how each unit operation will impact the next. So, we recommend optimizing one unit operation, then proceeding to optimize the next one in the workflow in sequential order.
Tip: Think about scalability and process closure from day one, because ideally your process will be transferred to a good manufacturing practices (GMP) environment. So, you want to make sure everything is sterile and that your process is closed. Also, remember to include stability ranges early on, such as room temperature holds.
View the event video for details on LVV downstream case studies, analytics, and more.
About Fast Trak Centre for Advanced Therapeutic Cell Technologies (CATCT)
This work was performed at the Fast Trak Centre for Advanced Therapeutic Cell Technologies (CATCT) in Toronto, Canada. This center was conceived in January, 2016 as a joint collaboration between Cytiva, CCRM, and the government of Canada. The center was tasked with developing the next generation of solutions for effective manufacturing of cell and gene therapy products to make these treatments more accessible to patients. In 2020 the center has grown to include more than 100 associates with expertise in process development and optimization, manufacturing, and many other specialties. Learn more about Fast Trak services for cell and gene therapy.